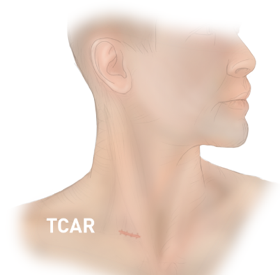
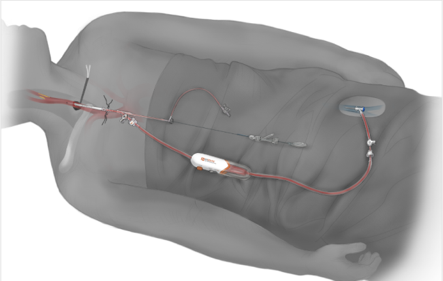
Oklahoma Heart Institute was the first in Tulsa to offer an innovative new treatment for patients at risk for stroke due to blockages in the neck arteries known as carotid artery disease. The minimally invasive procedure, called TransCarotid Artery Revascularization or TCAR, utilizes a new FDA-approved neuroprotection system that temporarily reverses blood flow in the artery during the procedure. Dangerous bits of plaque and blood clots that could dislodge and otherwise travel to the brain and cause a stroke are safely diverted away while a dedicated transcarotid stent is inserted to open and stabilize the blockage.
Prior to TCAR, the main treatment option for severe carotid artery disease was an open surgical procedure called carotid endarterectomy. The surgical technique allows for protection of the brain during the procedure, but the large incision leaves a visible scar the length of the neck and carries risks of surgical complications including bleeding, infection, heart attack, and cranial nerve injuries that can cause issues with swallowing, speaking, and sensation in the face.
You should be screened for carotid artery disease if you have:
- Weakness, numbness, tingling or paralysis of the arm, leg, or face on one side of your body
- Trouble swallowing
- Loss of eyesight or blurry eyesight in one eye
- Dizziness, confusion, fainting, or coma
- Unexplained slurred or garbled speech
Every year, 15 million people worldwide suffer a stroke, also known as a “brain attack”. Nearly six million die and another five million are left permanently disabled. Stroke is the second leading cause of disability globally. Carotid artery disease is estimated to be the source of stroke in up to a third of cases and there are 400,000 new diagnoses of carotid artery disease made every year in the United States alone.

and Adel M. Barkat, M.D., vascular surgery