Two new studies look at transcatheter aortic valve replacement (TAVR) as improving the survival rates of high-risk patients with severe aortic stenosis versus traditional surgical valve replacement and compare leading FDA-approved valves available, the Edwards Sapien XT and Medtronic’s CoreValve. Director of Structural Heart Disease and Transcatheter Aortic Valve Replacement at Oklahoma Heart Institute, Dr. Kamran Muhammad, shares what this recent news means for patients. “Overall, the recent data presented at the American College of Cardiology (ACC) Annual Scientific Sessions regarding transcatheter aortic valve replacement (TAVR) are very exciting and encouraging,” says Dr. Muhammad. “Broadly, the information and data from these landmark studies published in leading medical journals and simultaneously presented at the ACC show the important and growing role of TAVR in treating high-risk aortic stenosis patients. More specifically, these recent studies show that TAVR is superior to surgical aortic valve replacement in high-risk patients, as it results in lower mortality (lower risk of death) following the procedure.”
Patients who are high-risk for surgical valve replacement may be unable to undergo surgery due to age, history of heart disease, frailty or other health issues.
“This solidifies the role of TAVR in treating aortic stenosis in high-risk patients,” explains Dr. Muhammad. “The future for the treatment of aortic stenosis is bright, with ongoing refinements in TAVR, new technologies and expanding indications.”
Whereas some patients with severe aortic stenosis, who were high-risk for surgical valve replacement, faced a 50 percent likelihood they would not survive more than two years following the onset of symptoms, TAVR offers new hope right here at home. “Oklahoma Heart Institute is fortunate to have a highly-experienced and versatile TAVR program to serve the patients of Tulsa and NE Oklahoma,” shares Dr. Muhammad. “Our team performed the first TAVR in Tulsa in May 2012 and have subsequently performed more than 70 TAVR procedures in less than 2 years. Oklahoma Heart Institute also was the first center in Oklahoma to implant the recently FDA-approved Medtronic CoreValve on a commercial basis in February 2014. OHI’s TAVR program is the only program in the region to have access to both the FDA-approved transcatheter valves (Edwards Sapien and Medtronic CoreValve). As such, OHI remains the most experienced TAVR program in the region with superior outcomes with the goal of providing world-class care to our patients.”
Learn more about the TAVR program at Oklahoma Heart Institute here.
Edwards Sapien Valve
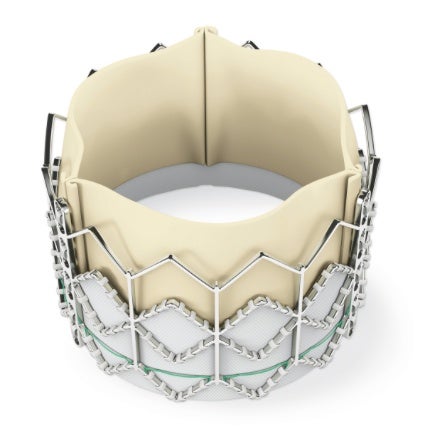
MedTronic CoreValve


